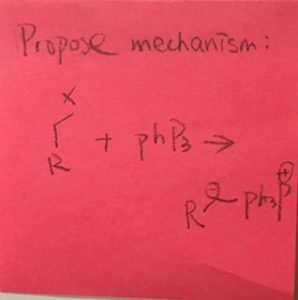
I’m sure you’re wittig (:P) enough to do the work on this ylide formation on your own, but if you need resources, here’s a handy reference: bit.ly/wittig-reaction. (BTW, it’s “PPh3” not “PhP3”). According to a chemist friend, it might help to think of it as a war: Electrons are the ammo. Some atoms have extra, and we call those lone pairs. Phosphorus is one of these. Some atoms are vulnerable, like the carbon atom that the X is attached to. The lone pair on the phosphorus attacks the carbon, kicks off the X (it’s usually chloride), and now it’s attached to that carbon. The X is now floating around naked and afraid, so it grabs a hydrogen off that carbon it just left and now it’s happy. This creates a double bond between the carbon and the phosphorus, which is the ylide, and which will now go on to do the Wittig reaction.

